Identify the Lewis Base in the Following Reaction
All of these are bases. Identify the Lewis acid and Lewis base in each of the following reactions.

Lewis Acids And Bases Chemistry Steps
H OH- H2O Lewis acid.
. Transcribed image text. PH 3 g H g --- PH 4 g Select one. Science Chemistry QA Library For each of the following reactions identify the acid and the base.
A Lewis acid is an electron pair acceptor. 112 Identify the Brønsted-Lowry acid-base pairs in each of the following equations. Arrhenius acid H2SO4 Arrhenius base KOH Neither NH3 HCl.
Check out a sample QA here See Solution star_border Students whove seen this question also like. Identify the Lewis acid in the following reaction. BF3 C5H5N - C5H5N - BF3 c.
In this case Fe3 gains electrons so Fe3 is the lewis acid. Advertisement Expert-verified answer 0 throwdolbeau. Identify the Lewis acid and the Lewis base in the following reactions.
PH 4 c. We review their content and use your feedback to keep the quality high. Who are the experts.
Details of Lewis Acids and Bases MP3 check it out. Weve got the study and writing resources you need for your assignments. Find step-by-step Chemistry solutions and your answer to the following textbook question.
Get How To Identify Lewis Acids And Bases MP3 Complimentary in Zai Airlinemeals uploaded by The Organic Chemistry Tutor. According to lewis theory of acid and bases. In some cases more than one definition may apply.
BF3 is a Lewis acid because of the fact this molecule has an incoplete octet of electrons. None of these is a base. Identify the Lewis acids and Lewis bases in the following reactions.
K 6H2O KH2O6 Lewis acid. Lewis acid is an electron pair acceptor. CuH2O4 2 aq 4 CN 1- aq CuCN4 2- aq 4 H2O l Given that Kc 1 for the reaction above which Lewis base is stronger.
In the reaction Cr3 aq 6H2O - Cr H2O63 aq Cr3 IS THE LEWIS ACID H2O is the Lewis base. Solution for For the following reaction identify the acid base conjugate acid and conjugate base. Identify the Lewis base in the following reaction.
SO3 H2O H2SO4. Identify the Lewis acid and the Lewis base in each of the following reaction 0309. Cl- BCl3 BCl4- Lewis acid.
Also indicate which acid- base definition Lewis Brønsted-Lowry applies. Ionic equilibrium class-12 1 Answer 1 vote answered Aug 31 2020 by Nilam01 357k points selected Sep 1 2020 by subnam02 Best answer i CaO CO2 CaO3 CaO Lewis base All metals oxides are Lewis bases CO2 Lewis acid CO2 contains a polar double bond. A Lewis acid is a compound or chemical that accepts a lone pair of electrons.
Identify the Lewis acid and Lewis base in each of the following reactions. Fe NO33 s6H2O lFe H2O63 aq3NO3 aq NH3 gHCl gNH4Cl s Drag the appropriate items to their respective bins. View the full answer.
Identify the Lewis acid and Lewis base in each of the following reactions. Identify the Lewis acid and the Lewis base in each of the following reactions. Chemistry College answered expert verified Using the Lewis concept of acids and bases identify the Lewis acid and base in each of the following reactions.
Experts are tested by Chegg as specialists in their subject area. Electron pair donor is base electron question_answer. CeSO2g H2Ol H2SO3aq What I tried.
н-ВН NH2 H-B-H O Both I I I- A. Identify the Lewis acid and Lewis base in the following reactions. A Lewis base is an electron pair donor.
Identify the Lewis acid and Lewis base in the following reaction. Fe3aq 6H2Ol Fe H2O63aq b. Start your trial now.
NaOH H20 Na I II III IV. Mathrm Fe left mathrm ClO _ 4 right _ 3 s 6 mathrm H _ 2 mathrm O I rightleftharpoons left mathrm Fe left mathrm H _ 2 mathrm O right _ 6. Identify the Lewis bases in the following reaction.
The how-to-identify-lewis-acids-and-bases have 2022-05-12 132118 and 874 MB. Using the Arrhenius concept of acids and bases identify the Arrhenius acid and base in each of the following reactions. For each of the following reactions identify the Lewis acid and the Lewis b 0107.
HgI2s 2I-aq HgI42- aq Expert Solution Want to see the full answer. The other problems of this type involved cations or anions so I was able to identify the cation as the Lewis acid and the anion as the. Hig2aq 4CN-aq-HgCN42-aq View Available Hints O Hg2 HgCN42- Submit Part B Classify each of the following as a Lewis acid or a Lewis base.
Identify the Lewis acid and the Lewis base in each of the following reactions. Co3 6 NH3 - CoNH363 d. SO2 HO- - HSO3-.
For the following reaction identify the Lewis acid. H2Ol CN-aq HCNaq OH-aq c. AlCl3 Cl- - AlCl4-b.
Identify the Lewis base in the following reaction. First week only 499. AlBr3 Br AIBr b.
Identify the Lewis acid and Lewis base among the reactants in each of the following reactions. Identify the Lewis acid and Lewis base in each of the following reactions. In the above equation Cr3 is accepting the lone pair of electrons.
100 4 ratings The ans. Mathrm SO _ 2 g mathrm H _ 2 mathrm O l rightleftharpoons mathrm H _ 2 mathrm SO _ 3 a q SO2 gH2 Ol H2 SO3 aq Explanation Verified Reveal next step Reveal all steps Create a free account to see explanations. A lewis acid accepts an electron pair while a lewis base donates an electron pair.
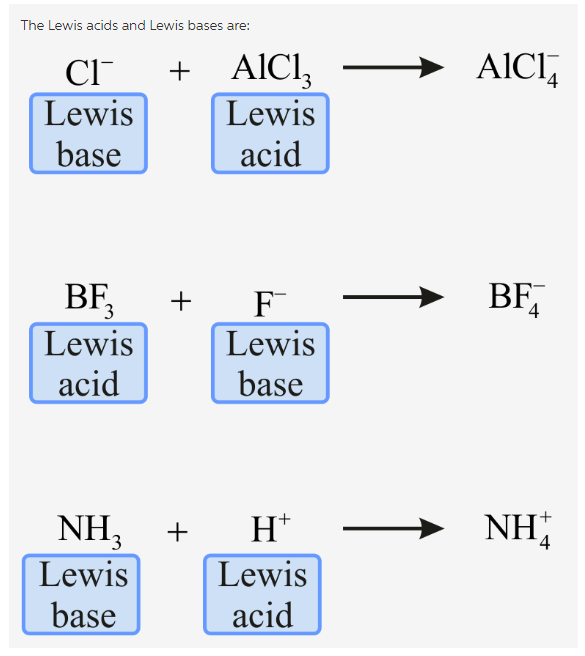
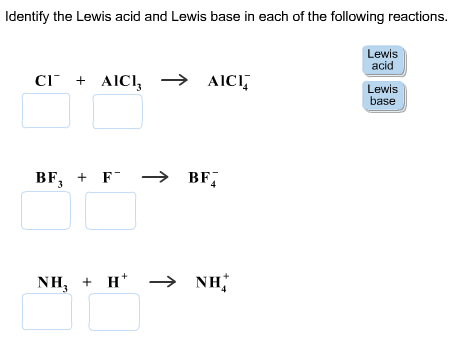
Cl- AlCl3 right arrow AlCl-4 BF3 F- right arrow BF-4 NH3 H right arrow NH4. 2KOH aqH2SO4 aqK2SO4 aq2H2O l NH3 gHCl gNH4Cl s Drag the appropriate items to their respective bins. Identify the Lewis base in the following reaction.
Identify The Lewis Acid And Lewis Base In Each Of The Following Reactions Home Work Help Learn Cbse Forum

14 2 Lewis Acids And Bases General College Chemistry Ii

Identify The Lewis Acid And Lewis Base In Each Of The Following Reactions Home Work Help Learn Cbse Forum

Comments
Post a Comment